Adsorption of Reactive Orange 13 onto Puffed Rice in Aqueous Solution
Abstract:- Adsorption of reactive orange 13(RO13) from aqueous solution onto puffed rice was investigated. Batch system and effects of effective parameters include pH, initial dye concentration, ionic strength and temperature was studied. It was found that maximum adsorption of reactive orange 13 onto puffed rice was at pH2, and extent of adsorption increases with increasing initial dye concentration and increasing temperature whereas the adsorption of RO13 decreases with the increase of ionic strength of the dye solution. Based on the result, it can be said pseudo-second order kinetic model is the best model for explanation of adsorption of RO13 onto puffed rice. The experimental adsorption data best fitted with the Langmuir’s isotherm model. Which indicates the adsorption is involved with monomolecular. The thermodynamic parameters such as adsorption enthalpy (H), entropy (S) and free energy (G) were evaluated by applying Van’t Hoff equation for the system which indicated the process involved with physisorption. The negative values of free energy (G) determined for this systems indicated that adsorption of Reactive Orange-13 was spontaneous at the temperatures under investigation (303-318 K). Puffed rice could be suggested as relatively efficient and low cost adsorbent for dye removal from textile wastewater.
Materials
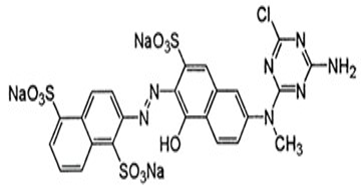
Puffed rice was collected from local market and was used without further purification although it was grained to make powder. The size distribution of puffed rice was determined using a laser scattering particle size analyzer (LDSA-2400A, Tonochi Computer Applications Japan) equipped with a dry dispersing apparatus (PD-10S, Tonochi Computer Applications Japan). Sample is a composed of large particles and small ones in diameter which is confirmed by the peaks around 32μm, 53μm and 355μm in frequency (Figure not given). The Reactive orange13 (RO13) was obtained from Sigma-Aldrich Germany and was used without purification. The chemical structure of RO13 is shown in Fig: 1. All other reagents and solvents were highest grade of purity. Deionized water was prepared by passing distilled water through a deionizing column (Branstead, Syboron Corporation, boston. USA).
Characterization
Zero point charge (pHZPC ) of puffed rice was determined. The FTIR spectra of reactive orange13 and puffed rice before and after reactive orange 13 adsorption were recorded in the frequency range of 400-4000cm-1 using spectrophotometer (Simadzu, Japan).
Batch adsorption experiments:
Batch sorption studies were carried out by agitating 25 mL of
dye solution of the desired concentration and 0.1g of the
puffed rice powder in 220 mL reagent bottle. Agitation was
performed at room temperature (30± 0.20C) with shacking
machine at a speed of 120r/min at different time intervals.
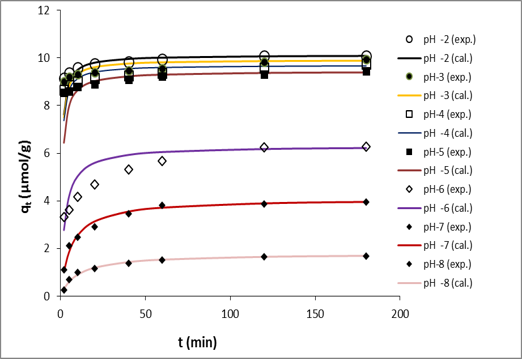
pH of the solution varied from 2 to 8. The dye adsorption
was determined spectrophotometrically (UV-160A, Shimadzu,
Japan)) by monitoring absorbance changes at the λmax
(488.0nm) of reactive orange 13 (RO13). Same method was
used for different initial concentration (65- 910 μmole/L) and
ionic strength (0.01M, 0.05M, 0.10M, 0.15M, 0.20M.
88.33μmol/L) of dye solutions and temperatures (30, 35, 40
and 45 0C) to study adsorption characteristics.The amount
of RO-13 adsorbed onto puffed rice qt (μmole/g) at any
time t and qe (μmole/g ) at equilibrium were determined
from the following relationships:
qt = V(C0 - Ct)/m (1)
and
qe = V(C0 – Ce)/m (2)
where C0 (µ mole/L), Ct (µ mole/L), Ce (µ mole/L), are the liquid-phase concentrations of RO-13 at initial, at any time t, and equilibrium, respectively; V (L) is the volume of RO-13 solution and m (g) is the amount of dry puffed rice power used. Desorption was also performed by producing complex of puffed rice and RO-13 and 0.1g of the complex was taken into 25 mL of 0.1 mole/L of NaOH solution and then performed batch adsorption experiments for 180 min. Reuse process were also performed to study the feasibility of puffed rice using as a good and commercially viable low cost adsorbent.
Determination of point zero charge (pHPZC )
Determination of Point Zero Charge (pHPZC) was done to determine the surface charge of Puffed Rice. It is important because this information helps us to explain the surface behavior of the adsorbent under certain pH. 0.1 M KCl solution was prepared and its initial pH was adjusted 2 to 8 (0.5 gap) using HCl and NaOH. Then 25ml of that solution was taken in the 125ml reagent bottle and 0.1g of Puffed rice added to each solution. These bottles were kept for 24 h and then final pH of the solutions was measured with a pH meter (410a Orion pH meter). Graphs were plotted between final pH and initial pH. (Fig is not shown) It was found that the pHPZC of puffed rice is 6.
FTIR Analysis
Which ensure that there is some interaction between OH and NH groups of puffed rice and S=O group of reactive orange13 dye
Conclusion
The amount of Reactive Orange 13 sorbed was found to vary with pH, initial dye concentration, ionic strength and temperature. pH2 is favorable for maximum adsorption. The amount of dye uptake (μmol/g) was found to increase with initial dye concentration and solution temperature, and found to decrease with increase in ionic strength. Rate controlling step of the adsorption process was governed by both of the intraparticle diffusion and film diffusion. The adsorption system studied can be well described by the second-order kinetic model. Thermodynamic activation parameter shows that the process is physisorption in nature. Experimental data were in good agreement with Langmuir isotherm. The negative value of the Gibbs energy (∆G) change of the adsorption indicates that the adsorption is spontaneous. The positive value of the enthalpy change (∆H) of the adsorption shows that the adsorption is an endothermic process. Reuse study shows puffed rice can be effectively used as a reusable adsorbent for the removal of Reactive Orange-13 from waste waters.
ACKNOWLEDGEMENT
Acknowedgemet to Jahangirnagar University,Department of Physical Chemistry and University Grant Commission, Bangladesh.
*Following blog is only for information purpose and Patchem Industries has neither endorsed nor performed such experiment on Orange H2R nor is liable for any misuse of informmation or images.*
